Learn more about how vaccine safety is monitored.
Last updated: June 23, 2023
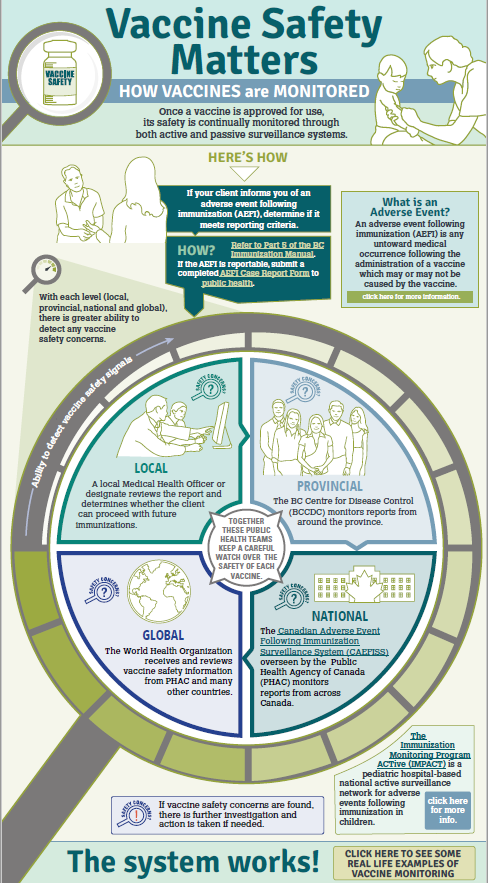
Health Canada has a thorough approval process that ensures the safety of the many vaccines and medicines we take routinely. This rigorous process is followed for all vaccines approved for use in Canada. Once a vaccine is approved and begins to be used, vaccine safety is continuously monitored to identify any serious adverse events.
Feeling worried or hesitant about new things is completely normal.
Approval happened quickly because Health Canada shortened the administrative and organisational process. The safety approvals did not change. The requirements for safety data in clinical trials are as strict as ever.
There’s always a small chance of side effects, for any medication or vaccine. Serious side effects are assessed in clinical trials. Thousands of people received the vaccine through these trials.
Once vaccines are approved and administered in a larger population, surveillance and evaluation systems continue to identify any side effects, known as adverse events following immunization (AEFI). Millions of people have now received the COVID-19 vaccines.
If these events happen, authorities investigate to identify whether the vaccine is directly responsible for the adverse event. Sometimes medical events occur within a few days of vaccination but are not related to the vaccine. If required, a vaccine can be withdrawn from the market and not used if there is a concern of harm. Health Canada can also issue warnings about specific risks in order to inform individuals.
B.C. monitors adverse events following immunization through the immunization surveillance system and reports to the
national and global safety surveillance systems.
[click to enlarge]
Getting vaccinated is important. Given the serious health consequences of COVID-19, the low likelihood of a serious reaction to a vaccine is outweighed by the benefits to you and your loved ones.
Vaccine safety surveillance is conducted for all vaccines including the COVID-19 vaccines under a regulatory framework at provincial and federal levels. All reports of COVID-19 vaccine adverse events following immunization (AEFI) are summarized.
Note: The report dated February 3, 2023 is the final report about adverse events following COVID-19 vaccines reported for the campaign which began in December 2020. While COVID-19 vaccination continues to be offered, the reporting of these events has declined dramatically since the start of the program. Regular reports about adverse events following immunization will be reported by BCCDC in relation to all vaccines, including COVID-19 vaccines, in the future.
- BC Report: AEFI with COVID-19 Vaccines - March 24, 2022
- BC Report: AEFI with COVID-19 Vaccines - March 10, 2022
- BC Report: AEFI with COVID-19 Vaccines - February 24, 2022
- BC Report: AEFI with COVID-19 Vaccines - February 10, 2022
- BC Report: AEFI with COVID-19 Vaccines - January 27, 2022
- BC Report: AEFI with COVID-19 Vaccines - January 13, 2022
- BC Report: AEFI with COVID-19 Vaccines - December 16, 2021
- BC Report: AEFI with COVID-19 Vaccines - December 02, 2021
- BC Report: AEFI with COVID-19 Vaccines - November 18, 2021
- BC Report: AEFI with COVID-19 Vaccines - November 4, 2021
- BC Report: AEFI with COVID-19 Vaccines - October 21, 2021
- BC Report: AEFI with COVID-19 Vaccines - October 7, 2021
- BC Report: AEFI with COVID-19 Vaccines - September 23, 2021
- BC Report: AEFI with COVID-19 Vaccines - September 16, 2021
BC Report: AEFI with COVID-19 Vaccines - September 9, 2021
BC Report: AEFI with COVID-19 Vaccines - September 2, 2021
-
BC Report: AEFI with COVID-19 Vaccines - August 26, 2021
-
BC Report: AEFI with COVID-19 Vaccines - August 19, 2021
-
BC Report: AEFI with COVID-19 Vaccines - August 12, 2021
-
BC Report: AEFI with COVID-19 Vaccines - August 4, 2021
-
BC Report: AEFI with COVID-19 Vaccines - July 29, 2021
-
BC Report: AEFI with COVID-19 Vaccines - July 22, 2021
-
BC Report: AEFI with COVID-19 Vaccines - July 15, 2021
-
BC Report: AEFI with COVID-19 Vaccines - July 8, 2021
-
BC Report: AEFI with COVID-19 Vaccines - July 2, 2021
-
BC Report: AEFI with COVID-19 Vaccines - June 24, 2021
-
BC Report: AEFI with COVID-19 Vaccines - June 17, 2021
-
BC Report: AEFI with COVID-19 Vaccines - June 10, 2021
-
BC Report: AEFI with COVID-19 Vaccines - June 3, 2021
BC Report: AEFI with COVID-19 Vaccines - May 27, 2021
BC Report: AEFI with COVID-19 Vaccines - May 20, 2021
BC Report: AEFI with COVID-19 Vaccines - May 13, 2021
-
BC Report: AEFI with COVID-19 Vaccines - May 6, 2021
-
BC Report: AEFI with COVID-19 Vaccines - April 29, 2021
-
BC Report: AEFI with COVID-19 Vaccines - April 22, 2021
-
BC Report: AEFI with COVID-19 Vaccines - April 15, 2021
-
BC Report: AEFI with COVID-19 Vaccines - April 8, 2021
Find national reports on vaccine safety from the Public Health Agency of Canada.
Health care professionals who need to report an adverse event following immunization should refer to this one-page resource on reporting adverse events following immunization.
In rare cases, people have experienced inflammation of the heart following immunization with a COVID-19 mRNA vaccine. Two conditions, called myocarditis and pericarditis, have occurred more often in younger adult and adolescent males and after the second dose. These events have been reported in B.C. at a rate of 1.5 per 100,000 doses of mRNA vaccine administered, and are seen more often after the second dose, and in males under 40 years of age. Most cases will have symptoms within a few days of vaccine receipt.
Typically, this condition after mRNA vaccines has been mild, with sometimes a brief hospital stay. People have recovered with or without treatment. Studies are ongoing to assess whether there are any residual effects. For further information, visit the
U.S. Centres of Disease Control.
The exact cause of these events is not known but is thought to be related to the immune response to the spike protein which is also important in immunity against COVID-19 virus. This is considered a safety signal of interest that is being monitored very closely.
These events have been seen internationally, and are being monitored in Canada with regular Public Health Agency of Canada reports here.
Symptoms of heart inflammation can include:
- Chest pain
- Shortness of breath
- Feeling of a rapid or abnormal heart rhythm.
If you experience these symptoms, seek medical attention right away. Inform the health care provider that you received a COVID-19 vaccine recently.
The benefits of vaccination continue to outweigh the risks and public health recommends that young people continue to get vaccinated against COVID-19. There are clear and significant benefits from mRNA vaccines in reducing severe disease, hospitalizations and deaths due to COVID-19 infections.
Rare cases of serious blood clots have been reported in individuals after they received the COVISHIELD or Janssen vaccines.
Please report any adverse events to your immunizer, healthcare provider, or doctor following your vaccination. Health care providers are trained to report these events to the correct channels to monitor vaccine safety.
If you have questions about side effects or a possible reaction to the vaccine, contact HealthLink BC by calling 8-1-1.
It is very rare for a vaccine to result in a permanent injury. Canada’s Vaccine Injury Support Program is a federal program that provides financial support to you if it is determined that you have experienced a serious and permanent injury after receiving a Health Canada-approved vaccine, administered in Canada on or after December 8, 2020. This includes COVID-19 vaccines as well as other vaccines approved by Health Canada. Financial support is also available to dependents of an individual who has died after vaccination. Learn more at vaccineinjurysupport.ca.
Information about adverse events is not shared between the Vaccine Injury Support Program and B.C.’s immunization program. If you submit a claim to the Vaccine Injury Support Program, please make sure you have also reported any adverse events to a healthcare provider in B.C. so that the event can be reported and vaccine safety can be monitored.
