Information and guidance on screening, diagnosis and management of patients with VITT/TTS
Last updated: October 20, 2023
Thrombosis with thrombocytopenia syndrome (TTS) (also known as vaccine-induced immune thrombotic thrombocytopenia or VITT), is a rare, idiosyncratic life-threatening condition that can arise after COVID-19 vaccination with viral vector vaccines such as AstraZeneca (AZ) or Johnson & Johnson/Janssen (JJ). Viral vector vaccines are no longer administered in Canada.
TTS has not been reported following COVID-19 vaccination with mRNA vaccines, such as Pfizer or Moderna, or protein subunit vaccines, the Novavax vaccine.
VITT/TTS is a condition characterized by
- symptom onset 4 to 28 days after exposure to AZ or JJ vaccine;
- platelet count less than 150 x 109/L; and
- thrombosis, especially in uncommon sites such as cerebral venous sinuses and abdominal veins (eg. portal vein), as well as arterial thrombosis.
Based on available data, the best estimate of the risk of VITT/TTS after vaccination with first dose AstraZeneca/COVISHIELD is 1 per 50,000 doses. The rate of TTS after the second dose of the AstraZeneca / COVISHIELD vaccine appears to be lower than with the first dose but has increased over time, with current estimates of approximately 1 per 600,000.
VITT/TTS resembles heparin-induced thrombocytopenia (HIT) in its mechanism, and diagnosis requires the presence of anti-PF4/heparin antibodies. Similar to HIT, early diagnosis is essential because patients can deteriorate rapidly. Currently, the recommended treatment is empiric initiation of intravenous immunoglobulin (IVIG) and a non-heparin anticoagulant.
Primary Care Outpatient Assessment
This guidance document provides a concise, point-of-care summary on assessing patients for VITT/TTS in the outpatient office. The emphasis is on vaccine history, high index of suspicion for thrombosis, getting a stat CBC when indicated, and sending a patient to the Emergency department if needed.
A patient-facing
COVID-19 vaccination aftercare handout is available about the possible side effects and symptoms to monitor in the days following vaccination (including symptoms of VITT/TTS).
Providers should report adverse events following immunization (AEFIs) to public health. This
infographic walks you through the steps of reporting an adverse event, answers common questions as to Who, What, When, Where, Why and How to report an AEFI, as well as providing a direct link to the
AEFI Case Report Form.
Clinical Care Pathway of VITT/TTS in British Columbia
This algorithm provides guidance to clinicians on how to screen, triage, diagnose and treat patients in a step-wise approach. Not all steps will be relevant to all clinicians, but an understanding of the expected level of care and the required steps is useful.
As the diagnosis of VITT/TTS requires a HIT ELISA test that is only available at St. Paul’s Hospital in Vancouver and at the Royal Jubilee Hospital in Victoria, rapid communication with hematologists and laboratory physicians is critical.
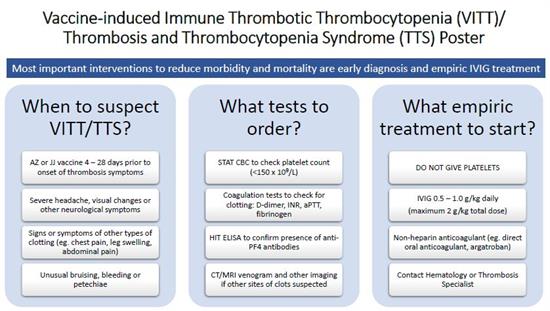
Open the VITT/TTS poster
This poster provides an overview of the main questions regarding VITT/TTS: When should VITT/TTS be suspected? What tests should be ordered? What empiric treatment should be started? This information is useful for all healthcare professionals who may be involved in other aspects of the patients’ care.
- Peak period of onset of thrombosis symptoms associated with VITT/TTS is 6 –14 days after vaccine
- Check if heparin/LMWH exposure within 1 –4 weeks (if positive, this could be heparin-induced thrombocytopenia [HIT])
- Most cases of VITT present with platelets <50 x 109/L and D-dimer is typically > 4x ULN
- MUST draw blood work for HIT testing and tell lab to arrange for VITT/TTS testing PRIOR to giving IVIG
- MUST order CT/MRI venogram to rule out cerebral venous sinus thrombosis (CVST) if bad headache or neurological symptoms
- Do not give platelet transfusion unless discussed with Hematology
- Call Hematology if vaccine-associated ITP or evidence of red cell fragments (schistocytes) on smear
- Treat as VITT/TTS unless ruled out with ELISA/SRA (IVIG + use non-heparin anticoagulant +/-steroids)
- If platelets <150 x 109/L and D-dimer highly elevated, BUT no thrombosis, consider IVIG only or prophylactic anticoagulation while awaiting HIT ELISA results
- If only thrombosis and platelets >150 x 109/L, start anticoagulant treatment (DOAC preferred) and monitor CBC to exclude early stage of VITT/TTS
- Platelet transfusion only if life-threatening bleeding or life-saving surgery indicated, and only AFTER IVIG has been given
- Consider fibrinogen replacement if less than 1.0 g/L and active bleeding
- Plasma exchange has been used in refractory cases (failure of IVIG and anticoagulant) with variable outcomes
- Review peripheral smear to rule out other causes of thrombosis + thrombocytopenia
- Sensitivity of HIT ELISA is considered sufficiently high to exclude VITT/TTS (optical density [OD] >2.0 in most cases)
- Non HIT ELISA assays have insufficient sensitivity and unknown specificity
- Serotonin release assay (SRA) at McMaster requires 2 red (or gold) top and 2 blue top tubes and
completed lab requisition
- Store plasma and serum for all suspected cases (vaccine timing +/-platelets <150 x 109/L +/-thrombosis)
- Other thrombophilia testing not required nor recommended

